Embryonic development, also known as embryogenesis, is a cornerstone in understanding the origins of life. But studying this wonder of complex and layered biological processes in humans faces significant challenges. Early stage human embryos are difficult to obtain. Then there are ethical questions about their use. This made it difficult for scientists to understand early human development.
However, advances in genetic engineering and molecular and cellular biology have led to the emergence of synthetic embolism, a subfield dedicated to replicating and studying embryonic development in a petri dish using human stem cells. . By offering new tools to explore the earliest enigmatic stages of human development, synthetic embolization can help researchers overcome the challenges of using real human embryos.
As a reproductive and developmental biologist, I develop stem cell models for embryogenesis. With these new models, researchers can better understand the conditions that affect human reproduction and development as well as maternal-fetal health, which could lead to new therapies .
Making human embryos from stem cells
Embryogenesis begins with the fertilization of an egg. This prompts the egg to divide rapidly into embryonic cells that soon form an inner cell mass that eventually develops into the fetus and an outer layer of cells that will give rise to the placenta.
Upon implantation in the uterus, the inner cell mass develops into three layers that will form all the tissues and organs of the human body. At the same time, the placenta begins to form as the embryo attaches itself to the uterine wall, a critical step for mother-fetal bonding. This attachment enables the transfer of nutrients, oxygen and waste between the mother and the foetus.
Synthetic embryology artificially recreates these stages of development using human pluripotent stem cells derived from human embryos or induced from adult human cells. Like early embryonic cells, these cells have the potential to develop into any type of cell in the human body. In carefully engineered laboratory environments, researchers can coax these cells to form multicellular structures that mimic various stages of embryonic development, including early organ formation.
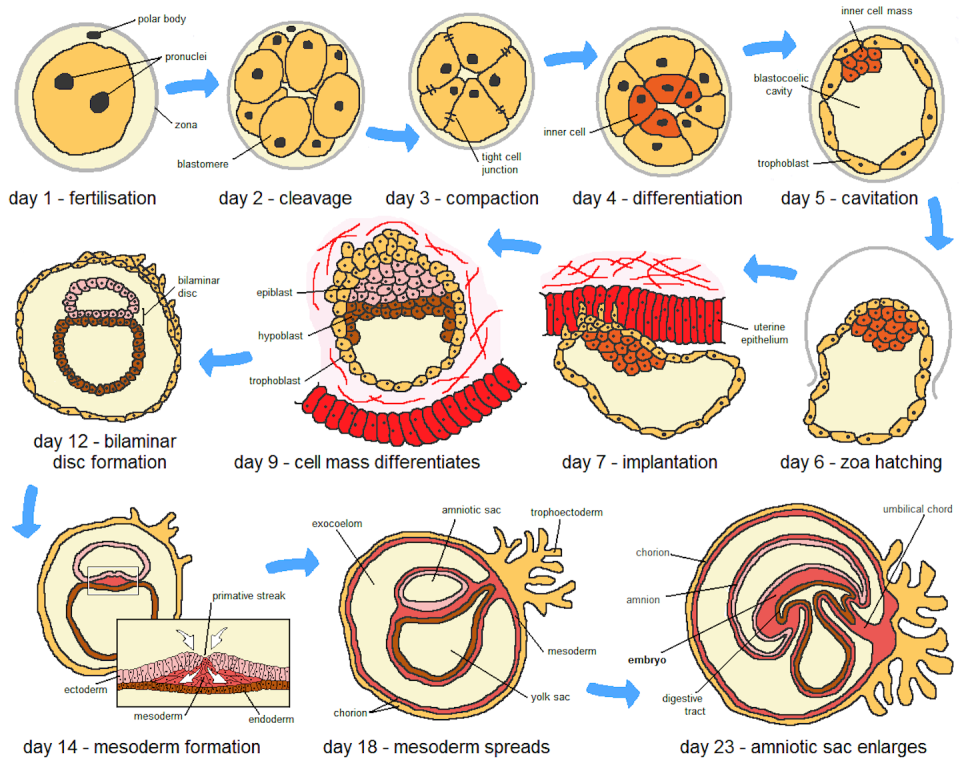
Researchers created the first human embryo model from embryonic stem cells in 2014. This pioneering model, also known as a gastruloid, captured key aspects of early human development and showed that scientists can drive multiple stem cells to form patterned layers resulting in the three germ layers and the outer layers of the embryo.
Gastruloids are easy to replicate and measure when studying early events in development. These 2D gastruloids can also help researchers identify and image embryonic cells. However, this model lacks the complex 3D structure and spatial cell interactions seen in natural embryogenesis.
Advances in human embryo models
Since the first gastruloid, the field has made significant progress.
Over the years, various models have been able to replicate various aspects of human embryogenesis, such as amniotic sac development, germ layer formation and body plan organization. Researchers have also developed organ-specific models for early organ development, such as a model that captures the key events of neural development and fetal lung organoids that mimic the process of lung formation.
However, none of these models fully captures the entire process of a single cell type developing into the complete structure of a complete embryo.
A significant breakthrough occurred in 2021 when several research groups succeeded in using human pluripotent stem cells with higher developmental potential to create blastoids, which are like early-stage embryos before implantation. Blastoids form in a similar way to human embryos, starting from just a few cells that multiply and organize themselves.
Because of the developmental and structural similarities between blastoids and embryos, they are useful for studying the early stages of how embryos form, especially before they attach to the womb. Blastoids can adhere to laboratory dishes and undergo further growth. They can also mimic embryo implantation in the uterus by integrating with the mother’s endometrial cells and developing into later embryonic stages after implantation.
Recently, researchers have succeeded in creating more complex models in the laboratory that mimic what happens when embryos attach to the womb. Two research teams used specially engineered cells to create structures similar to those of human embryos at about one week post-implantation. These models are also able to convert the cells into sperm and eggs in humans, reflecting what happens in natural development.
Another research group was also able to create a similar model from multiple stem cells without the need for genetic engineering. This model is able to mimic the later stages of development and the beginning of the formation of the nervous system.
Choosing the right models
In the evolutionary field of synthetic embryogenesis, no single model can perfectly capture all aspects of embryogenesis. As a result, the purpose is not to play God, to create life in a Petri dish, but to increase our understanding of ourselves. This goal emphasizes the importance of carefully selecting the model that best fits the specific research objectives at hand.
For example, my previous work focused on chromosomal abnormalities in early human development. Aneuploidy, or cells with an abnormal number of chromosomes, is a major cause of pregnancy loss. But scientific knowledge about how these abnormal cells affect pregnancy and fetal development is very limited.
Since gastruloids can effectively model these aspects of early development, this system may be suitable for studying aneuploidy in early development. It allows researchers to precisely track and analyze how aneuploid cells behave and how they influence developmental processes.
Using this model, my team and I discovered that cells with chromosomal abnormalities are more likely to mature into placental cells and likely to be eliminated during fetal cell development. This finding provides significant insight into why babies with a normal number of chromosomes can be born healthy even when aneuploidy is detected during pregnancy. Such discoveries are valuable for improving diagnostic and prognostic methods in prenatal care.
Future models that more fully replicate embryonic structures and more closely mirror biological events will not only advance understanding of the fundamentals of early development but will also have great potential to address clinical problems. Researchers can use them to model disease and develop drugs for early life or genetic conditions. These models are also invaluable for studying tissue formation in regenerative medicine. Creating embryo models from the patient’s own cells could allow researchers to study developmental genetics and help personalize treatments.
Crucial to progress in the field of synthetic embolization is unwavering adherence to ethical and regulatory standards. Crucially, these embryo models are neither synthetic nor actual embryos. The International Society for Stem Cell Research strictly prohibits the transfer of these embryonic models into the uterus of a person or animal. Although these models mimic certain aspects of the early stages of development, they cannot and will not develop like a human baby after birth. Basing research on justification and robust oversight will help ensure that the fabric of life is scientifically explored with respect and responsibility.
By embracing the complexities and potential of synthetic embolism, researchers are ushering in a new era in biological understanding and poised to solve the mysteries of life itself.
This article is republished from The Conversation, a non-profit, independent news organization that brings you reliable facts and analysis to help you make sense of our complex world. It was written by: Min Yang, University of Washington
Read more:
Min (Mia) Yang receives funding from the University of Washington