Cell migration, or how cells move in the body, is essential for normal body function and the progression of disease. Cell movement is what allows body parts to grow in the right place during early development, wounds to heal and tumors to metastasize.
For the past century, the way researchers understood cell migration was limited to the effects of biochemical signals, or chemotaxis, that instruct a cell to move from one place to another. For example, a type of immune cell called a neutrophil migrates towards areas in the body with higher concentrations of a protein called IL-8, which rises during infection.
In the last twenty or thirty years, however, scientists have begun to recognize the importance of the mechanical, or physical, factors that play a role in cell migration. For example, human mammary epithelial cells – the cells lining the milk ducts in the breast – migrate towards areas of increased stiffness when placed on a surface with a stiffness gradient.
And now, instead of focusing on the effect of the cells’ “solid” environment, researchers are turning to their “fluid” environment. As a trained theorist in applied mathematics, I use mathematical models to understand the physics behind cell biology. My colleagues Sean X. Sun and Konstantinos Konstantopoulos and I were among the pioneering scientists who discovered how water and hydraulic pressure affect cell migration through theoretical models and laboratory experiments. In our recently published research, we found that human breast cancer cell migration is enhanced by the flow and viscosity of the surrounding fluids, which clarifies one of the factors that influence how tumors grow.
How fluids affect cell migration
Cells in the human body are constantly exposed to fluids with different physical properties. Water is one such fluid that can guide cell migration. For example, we discovered that the way water flows across the membranes of breast cancer cells affects the way they move and grow. This is because the amount of water traveling in and out of a cell decreases or swells, which promotes movement by moving different parts of the cell.
The viscosity, or thickness, of body fluids varies from organ to organ, and from health to disease, and this can also affect cell migration. For example, the fluid between cancer cells in tumors is more viscous than the fluid between normal cells in healthy tissue. When we compared how fast breast cancer cells migrate in confined channels filled with normal viscosity fluid compared to high viscosity fluid, we found that cells in high viscosity channels counter intuitively increased by up to a significant 40% . This discovery was unexpected because the basic laws of physics tell us that inert particles should slow down in a high-viscosity fluid due to increased resistance.
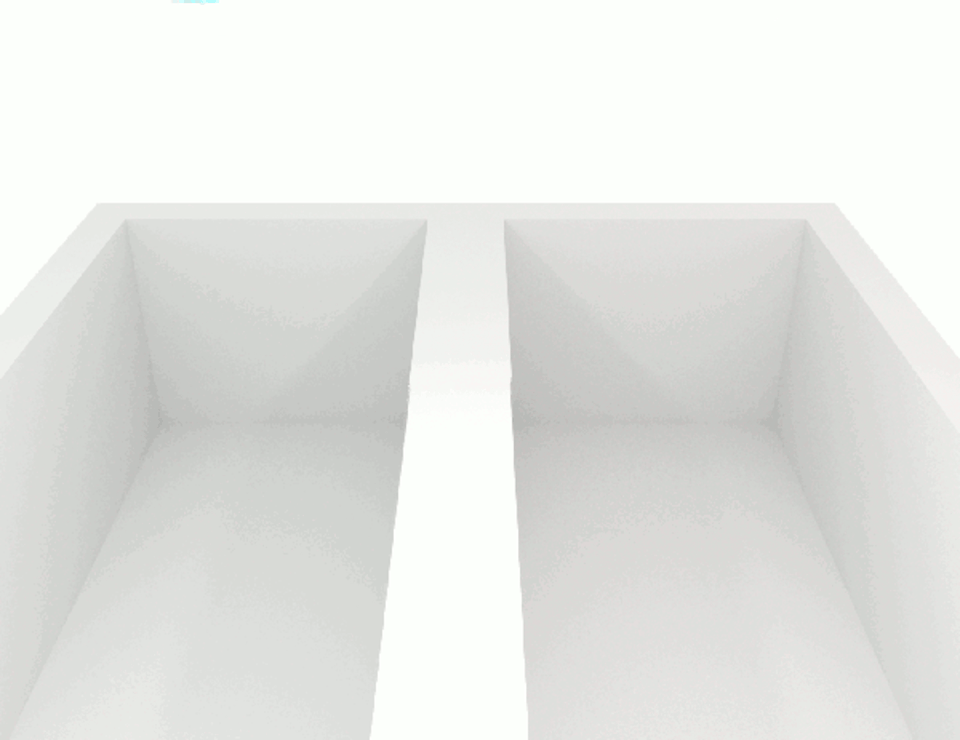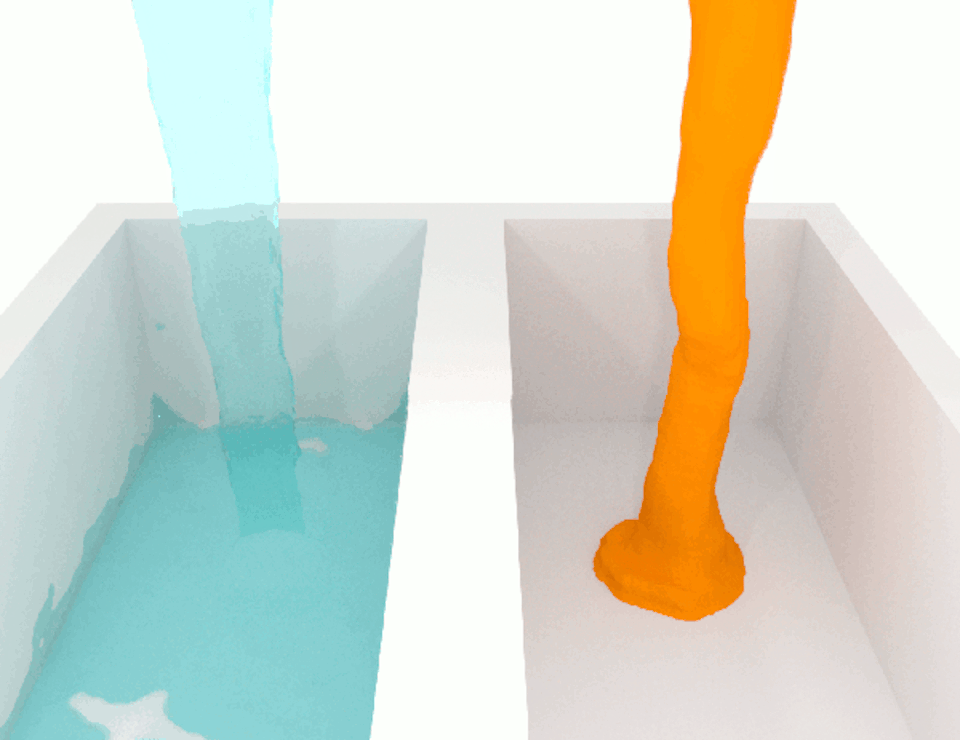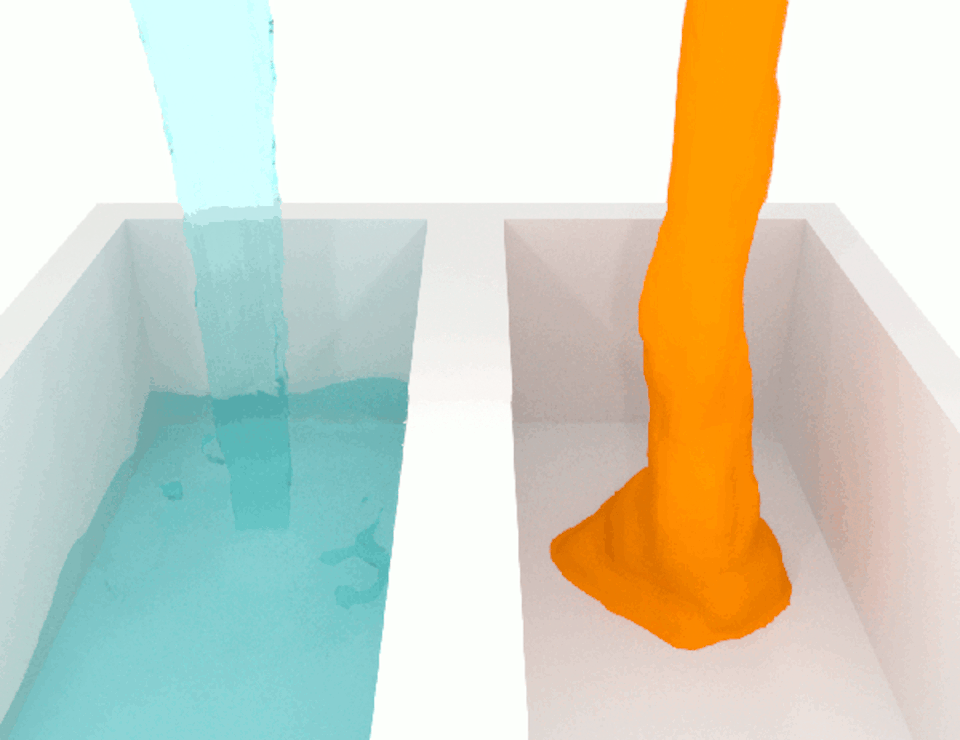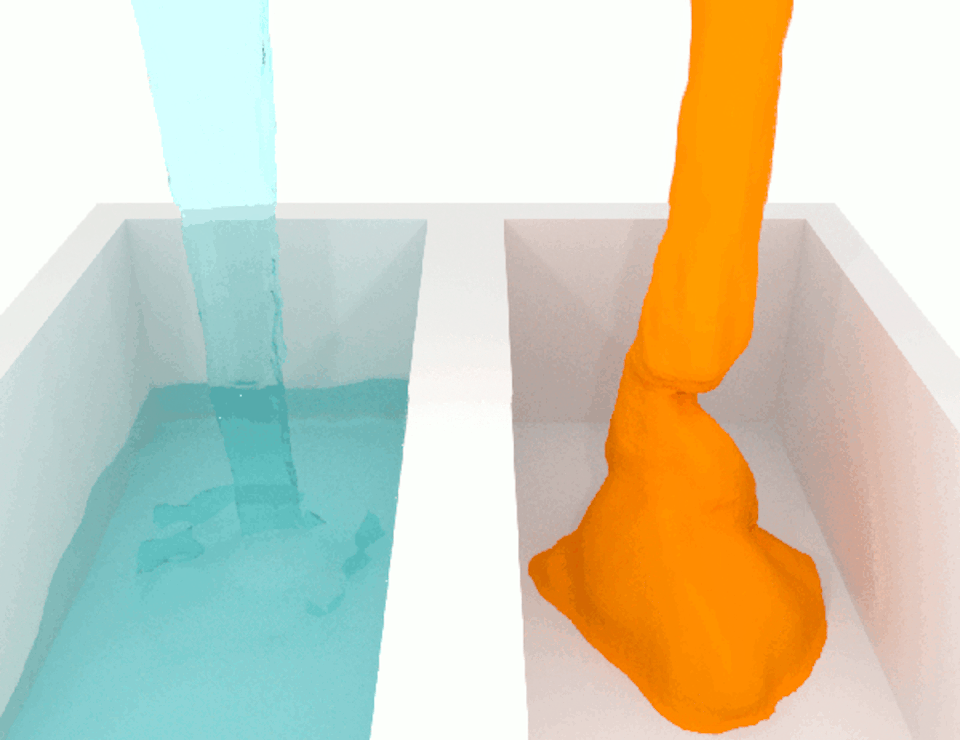
We wanted to figure out the mechanism behind this surprising result. So we identified which molecules were involved in this process, discovering a cascade of events that allows high-viscosity environments to enhance cell movement.
We discovered that a high-viscosity fluid first promotes the growth of a protein filament called actin, which opens channels in the cell membrane and increases water intake. The cell expands from the water, activating another channel that takes in calcium ions. These calcium ions activate another type of protein filament called myosin that stimulates the cell to move. This cascade of events prompts cells to change their structure and generate more force to overcome the resistance imposed by a high-viscosity fluid, which means that the cells are not inert at all.
We also found that cells retained a “memory” after exposure to a high viscosity medium. This meant that if we placed cells in a high viscosity medium for several days and then returned them to a normal viscosity medium, they would still move at a faster rate. How cells retain this memory is still an open question.
We then looked to see if our findings on viscous memory would hold true in animals, not just in Petri dishes. So we exposed human breast cancer cells to high viscosity medium for six days, then put them in normal viscosity medium. Then we injected the cells into chicken embryos and mice.
Our results were consistent: Cells that were pre-exposed to a high viscosity medium had an increased ability to leak into the surrounding tissues and metastasize compared to cells that were not pre-exposed. This finding indicates that fluid viscosity in the cell’s surrounding environment is a mechanobiological cue that promotes cancer cell metastasis.
Implications for cancer treatment
Cancer patients usually die not from the original source of the tumor, but from its spread to other parts of the body.
As cancer cells travel through the body, they move into spaces of varying fluid viscosity. Understanding how fluid viscosity affects the movement of tumor cells could help researchers find ways to better treat and detect cancer before it metastasizes.
The next step is to build imaging and analysis techniques to examine precisely how cells from different types of laboratory animals respond to changes in fluid viscosity. By identifying the molecules that control how cells respond to changes in viscosity, it could help researchers identify potential drug targets to reduce the spread of cancer.
This article is republished from The Conversation, a non-profit, independent news organization that brings you reliable facts and analysis to help you make sense of our complex world. It was written by: Yieng Li, Binghamton University, State University of New York
Read more:
Yieng Li receives funding from the National Science Foundation.